
Psychedelic Therapies Gain New Advocates
Written by Robin Berghaus.
Art by Matthieu Bourel
Marcus Capone served seven combat tours as a Navy SEAL — including missions with SEAL Team Six, the nation’s preeminent counterterrorism unit — before being medically retired in 2013. For years, he struggled to find effective treatments for his traumatic brain injuries, depression, and post-traumatic stress disorder.
Capone tried antidepressants, talk therapy, hyperbaric oxygen therapy, transcranial magnetic stimulation, and he visited several brain clinics. Nothing worked.
When Capone’s life began falling apart, he considered suicide. His wife was just as desperate. “I was going to lose him,” recalls Amber Capone.
As Amber searched for ways to help Marcus, she discovered other Veterans finding success with psychedelic therapies for their depression and PTSD. But those treatments are illegal in the United States – owing to decades of convoluted legal and regulatory practices.
Marcus would have to leave the country if he wanted to receive this life-saving treatment. A proud Veteran, he had never done illicit drugs. But absent other options, Marcus agreed. In 2017, he visited a Mexican clinic, where he was treated with ibogaine and 5-MeO-DMT, a toad’s toxin so potent some refer to it as “the God molecule.”
“The stress and anxiety went away,” says Marcus. “It changed my life forever.”
Psychedelic therapies were far more effective than anything else Marcus tried, inspiring the Capones to found Veterans Exploring Treatment Solutions (VETS). Since 2019, their non-profit based in Southlake, Texas has helped nearly 1,000 Veterans access psychedelic therapies in countries where they are legal or unregulated with a mission to end Veteran suicides.
Recently, the Capones shared their quest in the documentary, In Waves and War, that premiered in Telluride in August.

U.S. drug policy reform typically has an audience among progressives. But the newest advocates trend conservative. This unusually bipartisan coalition hopes psychedelic therapies will soon reach those who need them most.
A Right to Try
Shane Pennington ’10 is a litigator in Washington, D.C., who doesn’t think the Capones and the Veterans they help should have to leave the United States for life-saving healthcare.
A partner at Porter Wright, where he serves as co-chair of the firm’s Administrative and Regulatory Law Practice Group, Pennington and a legal team have worked pro-bono for four years so terminally ill patients can get treatments the U.S. Drug Enforcement Administration has criminalized.
Pennington represents Dr. Sunil Aggarwal, a Seattle palliative care physician treating patients like Erinn Baldeschwiler, who has stage 4 breast cancer. Pennington is fighting for Baldeschwiler to access psilocybin under federal and state Right to Try laws created for terminal patients who can’t wait years for drug approvals.
In 2018, the U.S. Food and Drug Administration declared psilocybin a breakthrough therapy with evidence that it may offer substantial improvement over available drugs for depression.
While psilocybin checks the boxes to qualify for Right to Try, there’s a catch. For over 50 years, psilocybin has been classified as a Schedule I drug with “no accepted medical use” – and that’s a sticking point for the DEA.
“Now here’s the thing,” says Pennington. “That definition is true of every experimental drug.”
In essence, with Schedule I drugs, it’s the DEA, rather than the FDA and doctors, determining whether and what drugs are medically viable. Pennington’s team petitioned the DEA in 2021 for an exemption and in 2022 to reschedule psilocybin from Schedule I to Schedule II in Aggarwal’s case. The DEA denied their petitions.
Aggarwal appealed the DEA’s denial of his rescheduling petition. The Ninth Circuit Court of Appeals ruled in Aggarwal’s favor in 2023, remanding the DEA to either clarify its pathway or to reevaluate Aggarwal’s petition on an open record. Troublingly, nearly a year after the court reversed the DEA’s denial, the agency still hasn’t sent the rescheduling petition to FDA for its review, despite psilocybin’s status as a breakthrough therapy.
In August, Pennington’s team returned to the Ninth Circuit to appeal the DEA’s denial of Aggarwal’s petition for an exemption. Aggarwal and his patients still haven’t heard from the DEA. They are awaiting a final ruling from the court.
“I have not met a single person who can give me a legitimate reason why this shouldn’t happen,” says Pennington. “What right does the DEA have to tell this doctor what he can and cannot do, while he is in good faith trying to heal his patients?”
Strange Bedfellows
Pennington is not an obvious choice to be on the vanguard of securing legal access to psychedelics. A vice president of the law school’s Federalist Society, managing editor of Texas Review of Law and Politics, and former clerk to D.C. Circuit Judge David Sentelle, Fifth Circuit Judge Jennifer Elrod, and U.S. District Judge Royce Lamberth ’67, the libertarian is no poster child for drugs.
But the battle to bring psychedelics out of the legal shadows and into mainstream medicine has become a bipartisan affair. Traditional conservatives, animated by deep convictions about liberty, are joining forces with progressives who share their outrage about governmental criminalization tactics that exacerbate suffering of Veterans and terminally ill patients.
And Texas has been at the forefront of this boundary-busting collaboration.
In 2021, Texas became the first state to publicly fund research for psychedelic therapies. Specifically, HB 1802 funds a clinical trial to study the effects of psilocybin for Veterans with PTSD.
Former Rep. Alex Dominguez (D-Brownsville) authored the bill and Rick Perry, former governor of Texas, helped garner bipartisan support. Both became advocates for psychedelic therapies after witnessing profound health transformations of Veterans they know.
In 2023, Rep. Dan Crenshaw (R-TX) worked across the aisle to pass the Douglas ‘Mike’ Day Psychedelic Therapy to Save Lives Act. Named after a decorated Navy SEAL who died by suicide, the congressional bill appropriated $10 million for studying psychedelic therapies for active-duty service members.
At a press conference with his Democratic cosponsors, including Rep. Alexandria Ocasio-Cortez (D-NY), Crenshaw called their team a “really wild coalition,” and said he couldn’t find anyone in Congress who opposed the bill.

Advocates for treatment reform note that between 17 and 44 Veterans take their lives every day. Nearly 150,000 have done so since 9/11. That’s more than 21 times the American lives lost in warzones over that same time.
Scheduled Out
For thousands of years, psychedelics have been used by Indigenous communities for religious ceremonies and healing rituals – from psilocybin mushrooms in Mexico to peyote-containing cacti in the Native American Church.
In the early 20th century, scientists began synthesizing psychedelics, including LSD and MDMA. Through the 1960s, psychedelic research for health conditions was mainstream with more than 40,000 research subjects and a handful of international psychedelic conferences.
Though results were promising, when LSD became the defining drug of the 1960s counterculture, backlash ensued. The Nixon-era Controlled Substances Act (CSA) of 1970 criminalized psychedelics, setting back research and development of effective medical therapies for decades.
The CSA created a scheduling system classifying drugs according to their medical use and risk of abuse. Schedule I drugs are defined as having “no accepted medical use and a high potential for abuse.” These substances are illegal to use, sell, and prescribe. Heroin, cannabis, LSD, MDMA, psilocybin, and nearly all psychedelic drugs are in this category.
While accessing Schedule I drugs is limited to research, applying for a license is a complex process that can take years to obtain. Most researchers avoid studying them altogether.
“That means we haven’t realized many medical benefits of psychedelics and other Schedule I drugs,” says Melissa Wasserman, professor and associate dean for research at the University of Texas School of Law. “When research is blocked, the lack of data contributes to a vicious cycle, making it difficult to get these drugs off Schedule I and into the medical community’s hands.”
All this informs Pennington’s legal efforts to help Baldeschwiler and her physician, Aggrawal.
Like many patients with terminal illnesses, Baldeschwiler experiences anxiety and depression while confronting agonizing end-of-life issues. Aggarwal, familiar with psilocybin as an end-of-life treatment, believes it might help alleviate her distress. When he asked if she would like to try it, Baldeschwiler said yes.
Aggarwal sought to enroll Baldeschwiler and other qualifying patients in a clinical trial, but none were accessible. So, he contacted a drug manufacturer to acquire psilocybin through Right to Try and treat them at his clinic. The manufacturer was willing to provide psilocybin, but by law required the DEA’s permission. When the DEA declined, Aggarwal sued.
“The DEA has not been willing to play ball,” says Wasserman. “Psilocybin is a breakthrough therapy. That’s a strong argument to allow Aggarwal’s petition. The DEA could make an exemption, as it has several times, including for the Native American Church to use peyote in its religious ceremonies.”
For Baldeschwiler, the DEA’s delay tactics add trauma to an already anxious time. “The DEA is hurting us. I should not have to turn to the underground or leave the country to get this medicine,” says Baldeschwiler.
The Department of Justice and its attorneys representing the DEA declined comment on pending litigation.
Opening Minds
Despite persistent legal obstacles, it’s clear why bipartisan support remains strong.
America faces a mental health crisis with nearly 50,000 people dying by suicide each year. Yet available antidepressant medications work for less than half of patients, and may elicit side effects, including suicidal thoughts.
University of Texas at Austin Dell Medical School assistant professor Dr. Greg Fonzo thinks psychedelics could help fill that unmet need. “In recent years, psychedelics have shown impressive evidence for treating patients’ depression and PTSD who are not served well by existing treatments,” he says.
Fonzo co-directs Dell Medical School’s Center for Psychedelic Research and Therapy. Among their projects, his team studies Veterans and family members whose loved ones died during their military service, each one seeking psychedelic therapies in Mexico and Peru for their depression and PTSD.
Before and after patients undergo treatments, Fonzo’s team takes blood samples, and performs brain scans and clinical assessments at Dell Medical School. Their goal is to decipher the underlying mechanisms of how psychedelics work and find ways to boost their efficacy.
Fonzo explains that psychedelics target brain receptors that produce psychological effects. Some patients report feeling more deeply connected with the universe. According to Fonzo, patients experience profound insights about themselves, which could be one way they process trauma.
What remains unknown is how psychedelics work on the brain for long-term improvements. Researchers believe psychedelics might promote neuroplasticity, the brain’s ability to form new connections.
Fonzo likens typical brain activity to a high school clique, in which certain students tend to talk to each other, while others remain excluded. Similarly, the brain segregates into communication hubs. “When people take a psychedelic, that structure tends to break down,” Fonzo says. “So, you see enhanced communication between areas of the brain that don’t normally communicate as much.”
One key benefit is that psychedelic therapies are relatively fast-acting. “We have seen striking improvements in some patients undergoing psilocybin or ibogaine treatments where people feel better within days,” says Fonzo. “Having that kind of treatment option is pretty profound.”
Decoding Psychedelics
These four substances have “breakthrough therapy” status from the FDA, meaning they show promise for treating serious conditions. The DEA says that all but ketamine have “no accepted medical use.” Who decides?
Psilocybin – Schedule I
Psilocybin, used for millennia, is a compound found naturally in certain mushrooms.
LSD – Schedule I
LSD is derived from the ergot fungus. Originally studied to treat alcoholism and trauma.
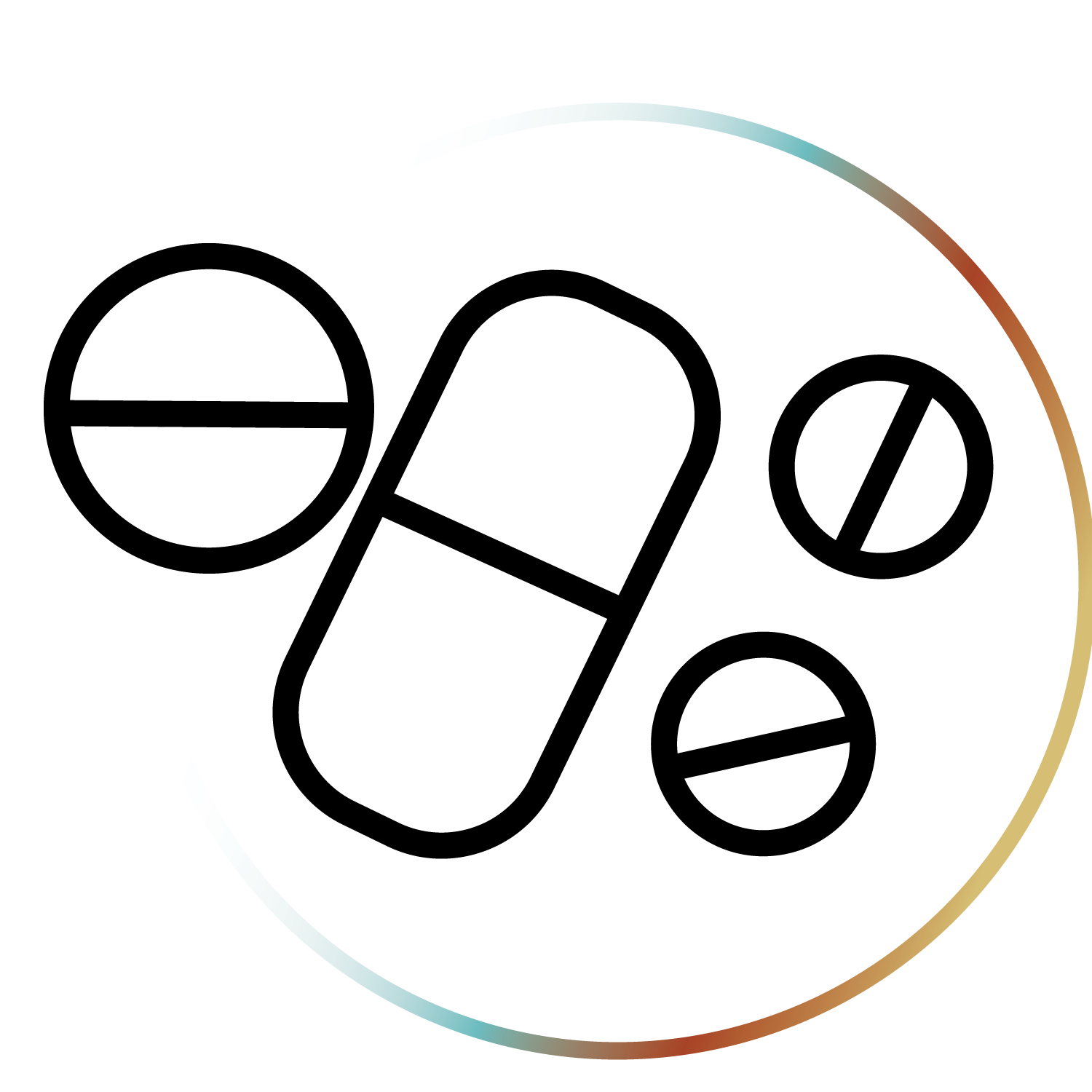
MDMA – Schedule I
MDMA increases empathy and bonding. Research shows promise in treating PTSD.
Ketamine – Schedule III
Ketamine is an anesthetic, prescribed off-label for depression.
Art: GreenTana / iStock
Common antidepressant medications, in contrast, take several weeks to show benefits, if at all.
But Fonzo notes that psychedelic therapies are not for everyone. Psychedelics tend to elevate heart rate and blood pressure. They can cause nausea, vomiting, and, in rare cases, episodes of paranoia.
Psychedelic therapies also aren’t as simple as taking a pill. The treatment process, lasting one to three months, involves a drug-therapy combination that considers patients’ set, setting, and integration.
Set, short for “mindset,” includes everything the patients bring to treatments – from their genetics to how they prepare mentally by considering risks and expectations.
Setting refers to the physical space designed for safety and comfort, where clinicians administer drugs and monitor patients throughout their psychedelic experiences. These sessions typically last from six to eight hours. Usually the next day, clinicians begin integration sessions to assess patients’ wellness and help them process what they’ve discovered as they move forward.
It’s unclear to what extent insurance will cover psychedelic therapies – each session is projected to cost thousands of dollars. If psychedelic therapies are approved, time and expenses may limit their accessibility.
Hope for Healing
Several psychedelic therapies are in clinical trials for patients with treatment-resistant depression, PTSD, migraines, substance use disorders, and an array of other conditions.
Lykos Therapeutics completed a Phase 3 clinical trial for its MDMA-assisted therapy. MDMA had been used in therapy until it became a Schedule I drug in 1985. If approved, MDMA would be the first new treatment for PTSD in over twenty years.
In August, the FDA declined to approve it, requesting another clinical trial. While the FDA did not (and is not required to) publicly share its recommendations for Lykos, in June the agency’s advisory panel cited issues. Panelists noted that participants could correctly guess whether they received MDMA or a placebo. Others expressed doubts about its efficacy, and concerns over the lack of long-term data collection on the potential for addiction and health risks.
Lykos intends to appeal the FDA’s denial.
More broadly, psychedelic-related initiatives are moving forward in a patchwork fashion across the country.
Voters in Oregon made history when they narrowly passed a 2020 ballot measure, making Oregon the first U.S. state to legalize psilocybin services. The law went into effect last year. Adults over 21 can now access psilocybin with trained facilitators at Oregon’s licensed service centers. Whether the DEA will intervene is unknown – accessing psilocybin outside of research is still a federal crime.
A 2023 JAMA Psychiatry article noted that 25 states proposed 74 bills and signed 10 into law. The bills propose a variety of measures from decriminalization, to research, to medical oversight of and licensure to prescribe or administer psychedelic therapies.
These efforts face an uphill battle. The time and expense of clinical trials and a legal maze of federal and state actors will continue to deter patients and providers – people like Marcus Capone, Erinn Baldeschwiler, and Dr. Sunil Aggarwal – from pursuing potentially life-saving treatments.
“We have a Veteran suicide epidemic, and where we draw the lines matters,” says Pennington. “It’s not just psychedelics, it is really about who is going to be accountable for solutions for healthcare – especially the mental health crisis – that plague our modern times.”
